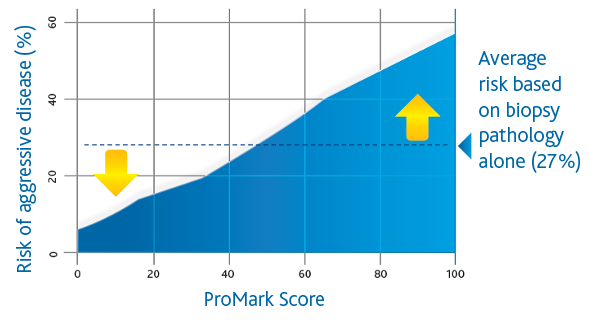
ProMark (Metamark Genetics, Inc., Durham, NC) is a biopsy-based Prostate Cancer prognostic assay that utilizes a multiplex immunofluorescence imaging platform to quantify the values of 8 protein biomarkers demonstrated to be relevant to Prostate Cancer aggressiveness in men with Gleason 3+3 and 3+4 Prostate Cancer. The biomarker values are incorporated into a risk score (ProMark Score; range: 1-100) indicating the likelihood of having high-risk disease. Lower ProMark scores are associated with lower risk of aggressive disease pathology (less than or equal to 3+4 and organ-confined disease), and higher ProMark scores are associated with a higher risk of aggressive disease pathology (upgrading or upstaging to Gleason =4+3 or non-organ-confined disease). The ProMark assay has also been shown to predict the likelihood of biochemical recurrence following definitive treatment, at the time of biopsy. The test has demonstrated the ability to predict Prostate Cancer aggressiveness regardless of the region from which the prostate biopsy was taken, a key feature and benefit given the considerable heterogeneity that exists in biopsied tissue.
ProMark was validated through a series of studies from initial proof of concept to final marker lock-down and validation trials involving more than 1200 patients in a 3-phase program. The assay should be used in conjunction with other laboratory and clinical information available to the clinician to support decisions on active surveillance or definitive treatment of men diagnosed with early-stage, clinically localized Prostate Cancer.